Schiff Base Examples: Exploring Their Versatile Uses
You might hear the name "Schiff" and, perhaps, your mind goes to health supplements or vitamins, which is understandable given brands like Schiff vitamins offering things like Move Free and Neuriva. That's a completely different area, though, as a matter of fact. Today, we're going to talk about something quite different yet equally fascinating: Schiff bases. These are a special kind of chemical compound, and they pop up in a surprising number of places, playing some very important roles. It's pretty cool, honestly, how one name can mean such different things.
So, what exactly are these chemical Schiff bases, you might wonder? Well, they're organic compounds with a specific structure, formed when an aldehyde or a ketone reacts with a primary amine. This reaction creates a carbon-nitrogen double bond, which is what gives them their unique properties. They're incredibly versatile, you know, and scientists have found countless ways to use them in various fields. Their ability to bind with metal ions, for example, makes them super useful in many applications.
This article will take a closer look at some compelling schiff base examples. We'll explore where you might find them, why they matter, and how they're making a real difference in areas like medicine, industry, and even how our own bodies work. It's truly amazing, basically, how a single chemical concept can have such a wide reach. Let's get into it.
Table of Contents
- What Exactly Are Schiff Bases?
- Why Do We Care About Schiff Bases?
- Common Schiff Base Examples in Everyday Life and Science
- Making Schiff Bases: A Quick Look
- The Future of Schiff Base Research
- Frequently Asked Questions
What Exactly Are Schiff Bases?
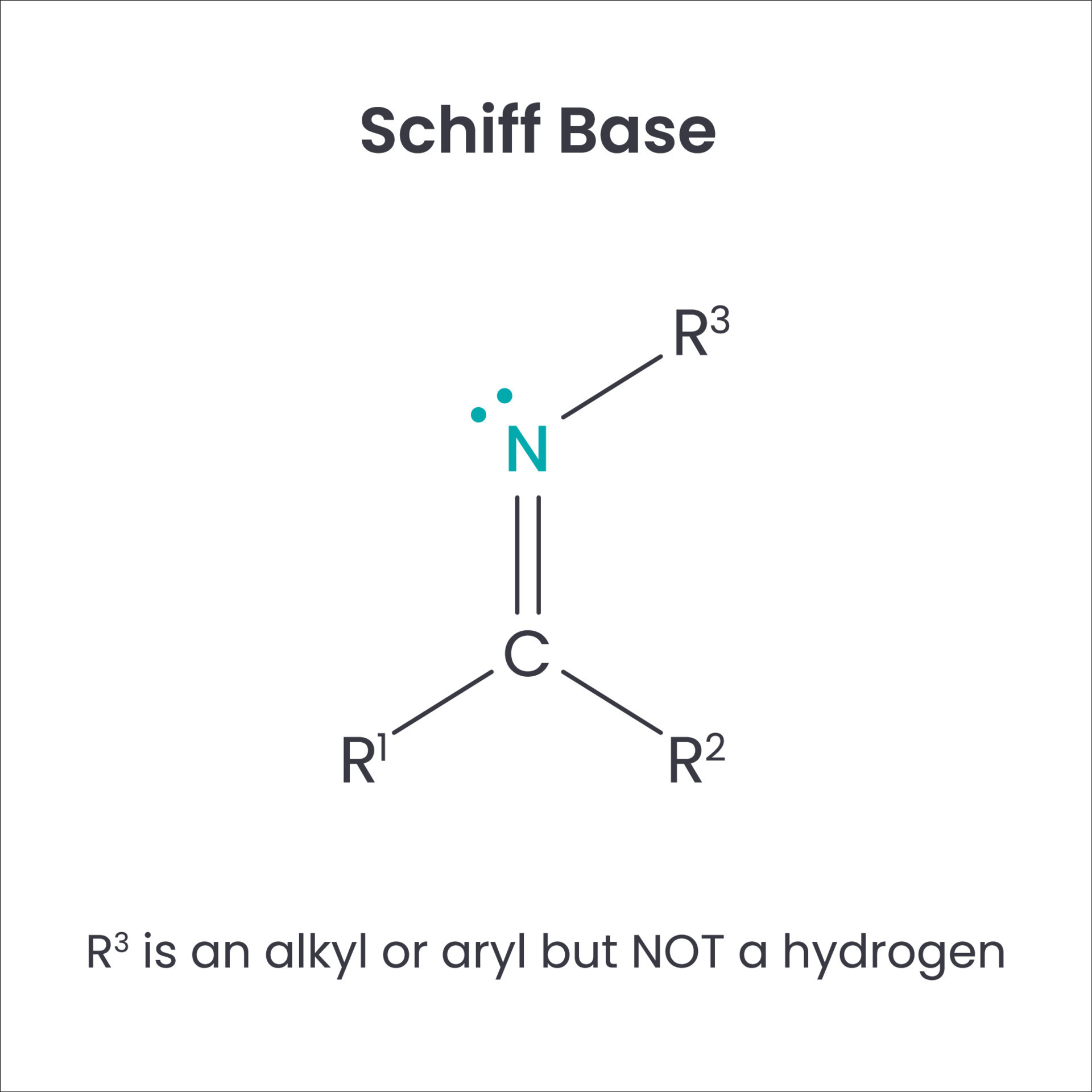
A Schiff base, sometimes called an azomethine or an imine, is a chemical compound that has a carbon-nitrogen double bond, where the nitrogen atom is connected to an alkyl or aryl group, but not hydrogen. They are formed through a condensation reaction, which is where two molecules join together and a smaller molecule, like water, is removed. This happens when an aldehyde or a ketone reacts with a primary amine. It's a pretty straightforward process, actually, which is part of why they're so widely studied.
The structure of a Schiff base, with that special carbon-nitrogen double bond, gives it particular chemical properties. This bond is quite reactive, meaning it can participate in other chemical reactions. It's also often stable enough to be isolated and used in various ways. You know, their unique electron distribution makes them really interesting to chemists.
These compounds are named after Hugo Schiff, a German chemist who first described them in the 19th century. So, while you might associate "Schiff" with things that support your immune system or joint health, this chemical term has a long history in organic chemistry. It's a fundamental concept, basically, that has led to many discoveries.
Why Do We Care About Schiff Bases?
Schiff bases are important for a few key reasons. One big reason is their ability to act as ligands. This means they can bond with metal ions, forming what are called coordination complexes. These complexes have a wide range of uses, often very different from the Schiff base itself. For example, some of these complexes can act as catalysts, speeding up chemical reactions, which is super helpful in manufacturing processes.
Another reason for their significance is their biological activity. Many Schiff bases, and their metal complexes, show promise in fighting off bacteria, fungi, and even certain types of cancer cells. This makes them a hot topic in drug discovery and development. You know, researchers are constantly looking for new ways to combat diseases, and these compounds offer a lot of potential.
Their relatively simple synthesis and the ease with which their properties can be adjusted also contribute to their popularity. By changing the starting aldehyde, ketone, or amine, scientists can create a vast array of Schiff bases with tailored characteristics. This versatility, in a way, makes them a go-to building block for many chemical endeavors.
In Medicine and Pharmaceuticals
Schiff bases have shown some really promising results in the medical field. For instance, many of them exhibit strong antimicrobial properties, meaning they can help fight off harmful bacteria and fungi. This makes them potential candidates for new antibiotics or antifungal medications, which is a big deal given the growing problem of drug resistance. Researchers are always, always, looking for fresh ways to tackle these issues.
Some Schiff base compounds also display anticancer activity. They can, apparently, interfere with the growth of cancer cells, sometimes even leading to their death. This area of research is quite active, with scientists exploring how these compounds might be developed into new chemotherapy drugs. It's a very complex field, but the initial findings are certainly encouraging.
Beyond fighting infections and cancer, Schiff bases are also being investigated for their anti-inflammatory effects. They might help reduce swelling and pain, which could be beneficial for conditions like arthritis. Furthermore, their ability to form complexes with metals means they could be used in diagnostic imaging or even in targeted drug delivery systems. So, you know, they really do have a lot of potential uses in health care.
In Industrial Applications
The industrial world also finds many uses for Schiff bases. One notable application is as corrosion inhibitors. They can form a protective layer on metal surfaces, preventing rust and degradation. This is incredibly important for pipelines, machinery, and other metal structures, saving industries a lot of money and ensuring safety. It's a rather clever way to extend the life of materials.
They are also widely used in the production of dyes and pigments. The specific chemical structure of some Schiff bases gives them vibrant colors, making them valuable in textiles, paints, and inks. Think about all the colorful products around you; some of that color might just come from a Schiff base. It's pretty neat, honestly, how chemistry brings so much color into our lives.
Moreover, Schiff bases act as catalysts in various industrial chemical processes. They can speed up reactions, making manufacturing more efficient and sometimes enabling reactions that wouldn't happen otherwise. This includes the production of fine chemicals, polymers, and even some agricultural chemicals. Their role in catalysis is a big reason why they are so important in large-scale production, basically.
In Biological Systems
It's fascinating to discover that Schiff bases aren't just man-made; they play crucial roles within living organisms too. A prime example is the role of pyridoxal phosphate (PLP), which is a form of vitamin B6. PLP forms a Schiff base with amino acids in many enzyme-catalyzed reactions. This temporary bond is absolutely vital for processes like amino acid metabolism, which is how our bodies build and break down proteins. It's a fundamental part of how we function, you know.
Another striking biological schiff base example is found in our eyes. The visual pigment rhodopsin, located in the retina, contains a Schiff base linkage. This linkage is formed between the aldehyde retinal and a lysine residue in the opsin protein. When light hits the eye, it causes a change in this Schiff base, triggering a cascade of events that leads to vision. It's how we see the world, really, a truly incredible chemical reaction happening constantly.
Beyond these specific examples, Schiff base formation is a common mechanism in many other biological processes involving enzymes and proteins. These reactions are often reversible, allowing for dynamic interactions that are essential for life. So, in some respects, Schiff bases are quietly working behind the scenes to keep us going, which is pretty amazing when you stop to think about it.
In Analytical Chemistry
In the world of analytical chemistry, Schiff bases are quite useful for detecting and quantifying various substances. Because many Schiff bases form distinctively colored complexes with metal ions, they can be used as indicators to identify the presence of specific metals in a sample. This is particularly handy in environmental monitoring, for example, to check for heavy metal contamination in water. It's a very precise way to spot things.
They are also employed in the development of chemical sensors. These sensors can detect a wide range of analytes, from gases to biological molecules, by changing color or showing a different signal when the target substance is present. This makes them valuable tools in research labs, industrial settings, and even for quick field tests. You know, being able to quickly identify what's in a sample is incredibly helpful.
The selectivity of some Schiff bases towards certain ions or molecules allows for very accurate measurements. This is important for quality control in manufacturing, for medical diagnostics, and for ensuring the safety of our food and water. So, in a way, they help us understand the composition of things around us, which is pretty fundamental to many scientific endeavors.
Other Interesting Schiff Base Examples
Beyond the more common applications, Schiff bases are also finding their way into other exciting areas. For instance, some have been explored for their potential in materials science, particularly in creating new types of polymers with unique properties. These might be stronger, more flexible, or have different electrical characteristics, leading to advanced materials for various uses. It's almost like building with custom LEGO bricks, but on a molecular level.
They are also being studied for their use in liquid crystals, which are materials that have properties between those of conventional liquids and solid crystals. Liquid crystals are essential components in many display technologies, like the screens on your phone or computer. The precise molecular arrangement of Schiff bases can contribute to the specific optical properties needed for these applications. This is a very specialized area, but quite important, apparently, for modern tech.
Furthermore, researchers are looking into Schiff bases for their potential in supramolecular chemistry, where molecules self-assemble into larger, ordered structures. This could lead to new ways of creating complex nanostructures or even molecular machines. The possibilities are truly vast, you know, as scientists continue to push the boundaries of what these compounds can do.
Making Schiff Bases: A Quick Look
The process of making a Schiff base is relatively straightforward, which is one reason for their widespread study and use. It typically involves a condensation reaction between an aldehyde or a ketone and a primary amine. This reaction usually takes place in a solvent, and sometimes a catalyst, like a weak acid, is added to speed things up. The water molecule formed during the reaction is often removed to push the reaction to completion. It's a rather elegant piece of chemistry, actually.
The simplicity of this synthesis means that chemists can easily create a huge variety of Schiff bases by simply choosing different starting materials. If you change the aldehyde or ketone, you get one type of Schiff base. If you change the amine, you get another. This ability to mix and match allows for the creation of compounds with very specific properties. This versatility, you know, is a huge advantage for researchers looking to design new molecules.
Because the reaction is fairly robust, it can be carried out under a range of conditions, making it accessible for many different research and industrial settings. This ease of preparation is a key factor in why we see so many different schiff base examples across various fields. It just makes them really practical to work with.
The Future of Schiff Base Research
The field of Schiff base research is still very active, with scientists constantly discovering new applications and properties. There's a lot of interest, for example, in developing more effective and safer drugs based on these compounds, especially for tackling antibiotic-resistant bacteria or hard-to-treat cancers. The potential for new medical breakthroughs is quite exciting, honestly.
Beyond medicine, researchers are exploring their use in advanced materials. Imagine new types of sensors that can detect tiny amounts of pollutants, or smart materials that can change their properties in response to light or temperature. Schiff bases might play a part in making these innovations a reality. It's a bit like building blocks for tomorrow's technology, in a way.
The ongoing exploration of their catalytic abilities also holds great promise for greener chemistry. If Schiff bases can help make industrial processes more efficient and less wasteful, that's a win for everyone. So, while we've covered many compelling schiff base examples today, it's clear that the story of these versatile compounds is still very much being written. There's so much more to learn about Schiff Vitamins on our site, and link to our product range, for example.
Frequently Asked Questions
What are Schiff bases used for?
Schiff bases are used for many things, including making medicines that fight bacteria or cancer, stopping metals from rusting in factories, creating bright dyes for clothes, and helping our bodies see and process food. They are also used in labs to find specific metal ions in samples. It's a very broad range of uses, honestly.
How are Schiff bases formed?
Schiff bases form when an aldehyde or a ketone molecule reacts with a primary amine molecule. This reaction typically removes a water molecule, creating a new carbon-nitrogen double bond. It's a type of condensation reaction, basically, and can happen quite easily in a lab setting.
Are Schiff bases stable?
The stability of a Schiff base can vary quite a bit depending on its specific chemical structure and the conditions it's in. Some are very stable and can be isolated and stored for a long time. Others are less stable and might break down easily, especially in the presence of water or strong acids. You know, it really just depends on the exact compound.
For more detailed information on imines and related compounds, you can check out resources like Britannica's entry on Imines.

Schiff base - Organic Chemistry - Science Forums

SchifFs base mechanisms - Big Chemical Encyclopedia
Schiff Base Biochemistry Functional Group science vector infographic